Low Circulating Natural Killer Cell Counts are Associated With Severe Disease in Patients With Common Variable Immunodeficiency
Lexature Silver
Mikae Ebbo, Laurence Gerard, Sabrain Carpentier, Frederic Vely, Sophie Cypowyj, Catherine Farnarier, Nicolas Vince, Marion Malphettes, Claire Fieschi, Eric Oksenhendler, Nicolas Schleinitz, eric Vivier
215 views
Abstract
Natural Killer (NK) cells have been shown to exert antiviral and antitumoural activities. Nevertheless most available data are derived from mouse models and functions of these cells in human remain unclear. To evaluate the impact of low circulating NK cell counts and to provide some clues to the role of NK cells in natural conditions, we studied a large cohort of patients with common variable immunodeficiency (CVID) included in a multicenter cohort of patients with primary hypogammaglobulinaemia. Patients were classified into three groups on the basis of their NK cell counts: severe and mild NK cell lymphopenia (< 50 and 50–99 × 106/L respectively), and normal NK cell counts (> 100 × 106/L). Clinical events were analyzed and compared between these three groups of patients. During study period, 457 CVID patients were included: 99 (21.7%) with severe NK cell lymphopenia, 118 (25.8%) with mild NK cell lymphopenia and 240 (52.5%) with normal NK cell counts. Non-infectious complications (57% vs. 36% and 35%), and, particularly, granulomatous complications (25.3% vs. 13.6% and 8.8%), were more frequent in patients with severe NK cell lymphopenia than in other groups. Invasive infections (68.7% vs. 60.2% and 48.8%), including bacteraemia (22.2% vs. 5.9% and 8.3%) and infectious pneumonia (63.6% vs. 59.3% and 44.2%), were also more frequent in this population. However, no difference was observed for viral infections and neoplasms. Low circulating NK cell counts are associated with more severe phenotypes of CVID, which may indicate a protective role of these immune cells against severe bacterial infections and other complications and non-redundant immune functions when the adaptive immune response is not optimal.
Article sourced from Pubmed: https://pubmed.ncbi.nlm.nih.gov/27211564/
This article did not undergo peer review on Lexature. Review history serves as an example for Lexaxture.
Highlights
CVID patients with low NK cell counts present increased rates of severe bacterial infections and granuloma.
Mortality appears to be especially high in CVID patients with both severe CD4+ T cell and NK cell deficiency.
NK cells could have non-redundant immune functions in patients with non-optimal adaptive immune response.
Forty years after their discovery, the functions of Natural Killer (NK) cells in natura remain poorly understood. Association studies linking clinical symptoms and defects in NK cell numbers or function are exceptional and poorly described in large cohorts of human patients. Our study analyzes the correlation between NK cell lymphopenia and clinical events in a large cohort of immunocompromised patients. We report the unexpected findings that severe invasive bacterial infections, especially bacteremia episodes, and non-infectious complications, especially granulomatous complications, are more frequent in common variable immunodeficiency patients with severe NK cell deficiency. These findings in natura highlights that NK cells may play a pivotal role in immunity in immunocompromised individuals, when the adaptive immune system is not optimal.
LaTeX Example:
1. Introduction
Natural Killer (NK) cells are cytolytic innate lymphoid cells (ILCs) that secrete an array of cytokines and chemokines (Spits et al., 2013, Vivier et al., 2011). Our knowledge of the biology of these cells has greatly increased over the last two decades, but the exact functions of human NK cells in vivo remain elusive. Indeed, the principal known functions of NK cells, such as the control of viral infections and cancers, were identified in studies on mice, and few data are available for humans (Vivier et al., 2011). One major obstacle to be overcome in studies of the role of NK cells in humans is the rarity of selective and well defined NK cell deficiencies (Jouanguy et al., 2013). Isolated NK cell deficiencies are exceptional, and full clinical phenotypes are not always available for the rare affected patients (Gineau et al., 2012, Hughes et al., 2012, Casey et al., 2012). It has been suggested that isolated NK cell lymphopenia is not associated with a particular clinical phenotype. This is the case in children with severe combined immunodeficiencies (SCID) with an NK − phenotype before hematopoietic stem cell transplantation (HSCT). The NK cell compartment is not successfully reconstituted after HSCT in a significant proportion of these patients (low NK cell count < 50 × 106/L), and the incidence of clinical events in these patients is similar to that in patients with normal NK cell counts (Neven et al., 2009). This may reflect redundancy between NK cell responses and other types of immune response, as such redundancy is frequently observed in immunity (Nish and Medzhitov, 2011). We therefore reasoned that the impact of an NK cell deficiency might be revealed by studies of patients presenting other immune defects. We explored the clinical phenotypes of patients with severe NK cell lymphopenia and compared them with those of patients with mild or normal NK cell counts, in a large cohort of patients with common variable immunodeficiency (CVID) from the French DEFI study.
2. Materials and Methods
DEFI is a French national study set up in 2004 to collect clinical data and biological specimens from adult patients with primary hypogammaglobulinaemia (Oksenhendler et al., 2008). The inclusion criteria in the DEFI study were adult patients with primary hypogammaglobulinaemia (serum IgG concentration < 5 g/L, or a serum IgA concentration < 0.7 g/L, or a serum IgM concentration < 0.4 g/L, or IgG subclass deficiency). The exclusion criteria were secondary hypogammaglobulinaemia and refusal to participate.
In total, 691 patients were included in the DEFI study. We present here the characteristics of 457 patients from 47 centers enrolled in the study between April 2004 and April 2013, all of whom were diagnosed with CVID. The diagnostic criteria used for CVID diagnosis were consistent with the European Society for Immune Deficiencies/Pan-American Group for Immunodeficiency criteria (Conley et al., 1999): (Spits et al., 2013) markedly low IgG levels (at least 2 SD below the mean for age) and markedly low levels of IgM, IgA, or both, (Vivier et al., 2011) a diagnosis of immunodeficiency after the age of two years, and (Jouanguy et al., 2013) no other known cause of hypogammaglobulinaemia. Screening of the genes encoding the CD40 ligand, the signalling lymphocyte activation molecule-associated protein, and Bruton's tyrosine kinase, was performed in male patients in the DEFI study, to exclude patients with X-linked hyper-IgM, X-linked lymphoproliferative disease, and X-linked agammaglobulinaemia, respectively, and AICDA (Activation-induced cytidine deaminase) was also sequenced to exclude patients with an autosomal recessive form of hyper-IgM from the CVID group.
The patients were enrolled in the study prospectively, and blood samples were collected, analyzed, and stored at inclusion. Clinical data were then collected retrospectively. The clinical file of each patient included a retrospective analysis of the patient's infectious, autoimmune, lymphoproliferative, and tumoural complications, and the patient's family medical history. A blood sample collected at inclusion was used for the determination of detailed T, B and NK cell phenotypes at the same centralized reference laboratory for all patients. All patients gave written informed consent for participation, including possible inclusion in genetic studies, and the study was approved by the local ethics committee. The clinical and biological database is centralized at the Department of Clinical Immunology of Saint-Louis Hospital in Paris. Clinicians were asked to inform the principal investigator of any significant clinical events during follow-up.
Patients were assigned to three groups on the basis of their NK cell counts (NK cells were defined as CD3− CD56+ CD16+ cells) at inclusion. Less than 5% of healthy controls have NK cell counts below 50 x106/L (Supplemental data Fig. S1, obtained in 145 healthy adults in our specialized NK cell laboratory). In another independent cohort of 52 blood healthy donors, median NK cell count was 185 × 106/L (IQR, 115–270), with no case below 50 × 106/L (data not shown). This last population was the reference population used in the reference laboratory of the DEFI study. We therefore used a cut-off of 50 NK cells × 106/L to define severe NK cell lymphopenia. Patients were also considered to have mild NK cell deficiency if they had NK cell counts between 50 and 99 cells × 106/L, and normal counts if their NK cell counts were ≥ 100 cells × 106/L at the time of evaluation. Clinical events and biological characteristics of patients with severe NK cell deficiency, mild NK cell deficiency and normal NK cell counts were analyzed and compared between groups.
Opportunistic infections (OIs) were consistent with the revised classification system of the manifestations of human immunodeficiency virus (HIV) infection and definition for AIDS from CDC (From the Centers for Disease Control and Prevention, 1993) and were referred to as OIs. Some atypical infections, such as pulmonary tuberculosis or recurrent herpes infections, are not included in these opportunistic infections and should be distinguished from “usual infections” during CVID (essentially ENT and respiratory bacterial infections by usual infectious agents). Instead, they were referred to as “unusual infections” (Supplemental data Table S1). Other clinical events were defined as previously described (Chapel et al., 2008, Chapel et al., 2012). Lymphoid hyperplasia was defined as any follicular hyperplasia or polymorph lymphoid infiltrate (without argument for lymphoma) occurring within lymphoid organ (lymphadenopathy, spleen, tonsils, cavum) or within extra-nodal/extra-lymphoid organ. Autoimmune disease was defined as any autoimmune disease excluding autoimmune cytopenia (rheumatoid arthritis, systemic lupus erythematosus, Sjögren syndrome, autoimmune thyroiditis, Biermer disease, type 1 diabetes, inflammatory myositis…). Finally enteropathy was defined as any cause of recurrent acute diarrhea or chronic diarrhea, including celiac-like villous atrophy and Crohn's-like inflammatory bowel disease. Documented granuloma, lymphoid hyperplasia or lymphoma in the gut were not included in this category but in the corresponding ones (respectively “Granuloma”, “Lymphoid hyperplasia” and “Lymphoma”).
Descriptive analysis used medians with interquartile range (IQR) values. Statistical comparisons between the 3 NK groups (< 50, 50–99, ≥ 100) were based on the non-parametric Kruskal–Wallis test for continuous variables and were based on Pearson's chi-square test or Fisher's exact test when appropriate for comparison of proportions of patients in multiple groups. Given the large list of clinical and biological characteristics compared between the 3 NK groups, we report Benjamini–Hochberg adjusted P values (Pc), which maintains the false discovery rate at the nominal alpha 0.05 level (Benjamini–Hochberg, 1995). Overall survival (OS) was calculated from inclusion in DEFI study until the last visit or death from any cause. Follow-up ended in April 2014. Survival was estimated by the Kaplan–Meier product-limit method. For OS comparisons, the groups considered were severe, mild and no NK cell lymphopenia, and then a second analysis was performed in 4 groups, as a function of NK cell and/or CD4+ T cell deficiency, and the log-rank test was used. Statistical analyses were performed with STATA Statistical Software version 11.1 (Stata Corp., College Station, TX, USA).
The DEFI study was funded by a national program for clinical research (PHRC 2005) and by the French National Centre for Hereditary Immune Deficiencies (CEREDIH), the Laboratoire Français du fractionnement et des Biotechnologies (LFB) and Baxter BioScience.
3. Results
3.1. Patient Population, Clinical Events and Treatments
Within the DEFI study, 466 patients were diagnosed with CVID, and NK cell counts at inclusion were available for 457 of these patients. We found that 99 of these 457 patients (21.7%) had severe NK cell lymphopenia (< 50 × 106/L), 118 (25.8%) had mild NK cell lymphopenia (50–99 × 106/L) and 240 (52.5%) had normal NK cell counts (≥ 100 × 106/L) (Fig. 1). The demographic and diagnostic characteristics of these three groups of patients are presented in Table 1. Sex ratio, median age at onset, at diagnosis and at evaluation, and the proportion of patients diagnosed before the age of 15 years were similar in these three groups. A trend towards a higher prevalence of consanguineous families was noted in the severe NK cell lymphopenia group (8·3% versus 4·4% and 5%, respectively).
Fig. 1.
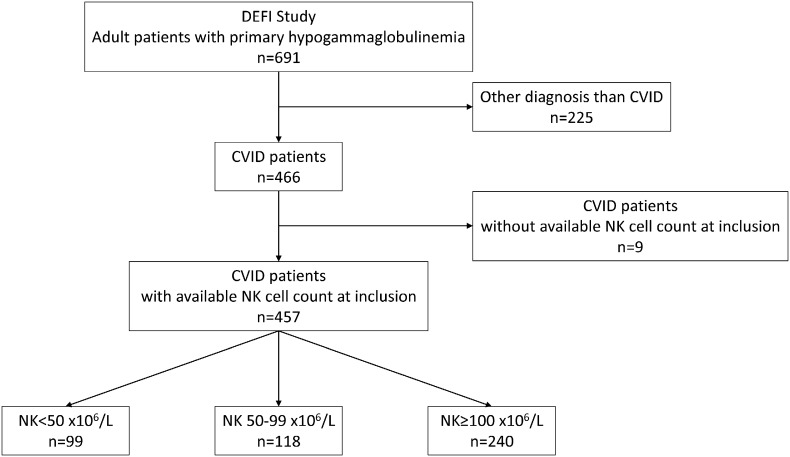
Flow chart of the “NK-DEFI” study.
Table 1. Demographic, diagnostic and therapeutic characteristics of three groups of patients with CVID defined on the basis of NK cell counts (< 50, 50–99 and ≥ 100 × 106/L).
Demographic, diagnostic and therapeutic characteristics | NK < 50 | NK 50–99 | NK ≥ 100 | Pc |
|---|---|---|---|---|
Sex, n (%) male patients | 49/99 (49.5) | 46/118 (39.0) | 100/240 (41.7) | ns |
Patients with known consanguinity, n (%) | 8/93 (8.6) | 5/110 (4.5) | 12/229 (5.2) | ns |
Age at onset (years) | 19.8 [11.7–34.8] | 22.5 [11.5–36.0] | 21.5 [10.4–38.1] | ns |
Age at diagnosis (years) | 34.9 [24.0–46.1] | 34.3 [21.6–49.1] | 36.8 [25.0–52.6] | ns |
Age at evaluation (years) | 44.1 [29·6–55.9] | 40.5 [27.7–55.3] | 44.8 [33.8–57.8] | ns |
Patients with diagnosis before the age of 15 years, n (%) | 8/99 (8.1) | 14/118 (11.9) | 24/240 (10.0) | ns |
Time from onset to diagnosis (years) | 9.3 [2.4–17.8] | 6.0 [1.2–15.1] | 6.5 [1.1–18.5] | ns |
Ig replacementa, n (%) | 86/99 (86.9) | 88/117 (75.2) | 157/240 (65.4) | < 10− 3 |
Splenectomy, n (%) | 9/99 (9.1) | 5/116 (4.3) | 24/240 (10.0) | ns |
Corticosteroid use during disease course, n (%) | 15/99 (15.2) | 13/113 (11.5) | 21/235 (8.9) | ns |
Immunosuppressive drug use during disease course, n (%) | 2/99 (2.0) | 3/115 (2.6) | 6/237 (2.5) | ns |
Note. For ages at onset, diagnosis, and evaluation, and time to diagnosis, the median is shown [with 25th and 75th percentiles]. All P values were adjusted by Benjamini–Hochberg procedure (Pc); ns = not statistically significant.
aIg replacement at evaluation in the DEFI study (corresponding to diagnosis for a part of patients).
Among these 457 patients, 275 patients (60.2%) presented infections only, whereas 182 patients (39.8%) presented both infections and other disease-related complications. The principal clinical events in CVID patients, according to their NK cell counts, are presented in Table 2. The prevalence of other disease-related complications (i.e. noninfectious complications) was higher in the group with severe NK cell lymphopenia than in the other two groups (57% vs. 36% and 35%, respectively; Pc = 0.02) (Fig. 2). The prevalence of tumours, including both solid tumours and lymphoma, was not significantly higher in the group with severe NK cell lymphopenia (19.2% vs. 16.9% and 14.2%, respectively), whereas granulomatous complications (25.3% vs. 13.6% and 8.8%, respectively), lymphoid hyperplasia (35.4% vs. 24.6% and 19.2%, respectively), enteropathies (41.4% vs. 28% and 24.2%) and autoimmune cytopenia (25.3% vs. 12.7% and 14.6%) appeared to be more frequent in the group with severe NK cell lymphopenia than in the other groups, but after adjustment for multiple testing by Benjamini–Hochberg correction, only the prevalence of granulomatous complications was found to be significantly higher in the group with severe NK cell lymphopenia (Pc < 0.001) (Fig. 2).
Table 2. Clinical events in three groups of patients with CVID defined on the basis of NK cell counts (< 50, 50–99 and ≥ 100 × 106/L).
Clinical events | NK < 50 | NK 50-99 | NK ≥ 100 | Pc |
|---|---|---|---|---|
Infections only, % (n) | 43.4 (43/99) | 63.6 (75/118) | 65.4 (157/240) | 0.02 |
| ||||
Other disease-related complications | ||||
Tumours, % (n) | 19.2 (19/99) | 16.9 (20/118) | 14.2 (34/240) | ns |
Lymphoid hyperplasia,a % (n) | 35.3 (35/99) | 24.6 (29/118) | 19.2 (46/240) | ns |
Granuloma, % (n) | 25.3 (25/99) | 13.6 (16/118) | 8.8 (21/240) | < 10− 3 |
Autoimmune disease,b % (n) | 13.1 (13/99) | 17.8 (21/118) | 16.7 (40/240) | ns |
Autoimmune cytopenia, % (n) | 25.3 (25/99) | 12.7 (15/118) | 14.6 (35/240) | ns |
Enteropathy,c % (n) | 41.4 (41/99) | 28.0 (33/118) | 24.2 (58/240) | ns |
| ||||
Infectious manifestations | ||||
Unusual infections, % (n) | 20.9 (18/86) | 27.4 (31/113) | 22 (52/236) | ns |
Opportunistic infections, % (n) | 10.1 (10/99) | 3.4 (4/118) | 5.8 (14/240) | ns |
Viral infections, % (n) | 18.2 (18/99) | 22.9 (27/118) | 16.7 (40/240) | ns |
Digestive infections, % (n) | 29.3 (29/99) | 19.5 (23/118) | 18.3 (44/240) | ns |
Invasive infections, % (n) | 68.7 (68/99) | 60.2 (71/118) | 48.8 (117/240) | 0.05 |
Infectious pneumonia, % (n) | 63.6 (63/99) | 59.3 (70/118) | 44.2 (106/240) | 0.02 |
Sepsis, % (n) | 22.2 (22/99) | 5.9 (7/118) | 8.3 (20/240) | < 10− 3 |
Meningitis, % (n) | 8.1 (8/99) | 5.1 (6/118) | 7.5 (18/240) | ns |
Note. All P values were adjusted by Benjamini–Hochberg procedure (Pc); ns = not statistically significant.
aLymphoid hyperplasia: any follicular hyperplasia or polymorph lymphoid infiltrate (without argument for lymphoma) occurring within lymphoid organ (lymphadenopathy, spleen, tonsils, cavum) or within extra-nodal/extra-lymphoid organ;
bAutoimmune disease: autoimmune diseases excluding autoimmune cytopenia (rheumatoid arthritis, systemic lupus erythematosus, Sjögren syndrome, autoimmune thyroiditis, Biermer disease, type 1 diabetes, inflammatory myositis…);
cEnteropathy: any cause of recurrent acute diarrhea or chronic diarrhea, including celiac-like villous atrophy and Crohn's-like inflammatory bowel disease. Documented granuloma, lymphoid hyperplasia or lymphoma in the gut were not included in this category but in the corresponding ones (respectively “Granuloma”, “Lymphoid hyperplasia” and “Lymphoma”).
Fig. 2.
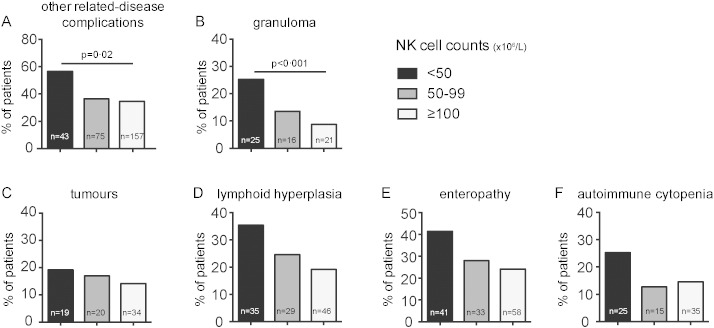
Proportion of CVID patients with “other disease-related complications”, i.e. non-infectious complications, according to NK cell count. Percentage of patients with A. other disease-related complications, B. granuloma, C. tumours, D. lymphoid hyperplasia, E. enteropathy and F. autoimmune cytopenia. In black, patients with severe NK cell deficiency; in grey, patients with mild NK cell lymphopenia; and in white, patients with normal NK cell counts. The statistical tests were two-tailed and the P values were then adjusted for multiple testing, by Benjamini–Hochberg procedure, for each type of complications (B to F).
The proportions of patients with different types of infectious events in each group are presented in Fig. 3. Viral infection episodes were equally frequent in each of the groups (20.2%, 22.9% and 17.1%, respectively). No susceptibility to a particular type of viral infection, such as Herpes viruses or Human papillomavirus, was noted in the group with severe NK cell lymphopenia (Supplemental data Tables S1 and S2). Opportunistic infections, consistent with the revised classification system of the manifestations of human immunodeficiency virus (HIV) infection (From the Centers for Disease Control and Prevention, 1993), were observed in 10 (10.1%), 4 (3.4%) and 14 (5.8%) patients from the groups with severe NK cell lymphopenia, mild NK cell lymphopenia and normal NK cell counts, respectively (Supplemental Table S2). Unusual infections, defined as atypical infectious events not consistent with manifestations of HIV infection, were found in 18 (20.9%), 31 (27.4%) and 52 (22%) patients from the groups with severe NK cell lymphopenia, mild NK cell lymphopenia and normal NK cell counts, respectively (Supplemental data Table S1). Severe infectious complications were grouped together to form an “invasive infections” category, including septicemia, pneumonia and meningitis. Invasive infections were more frequently observed among patients with severe and mild NK cell lymphopenia than among patients with normal NK cell counts (68.7% vs. 60.2% and 48.8%; Pc = 0.05; Fig. 3). Pneumonia (63.6% vs. 59.3% and 44.2%; Pc = 0·02) and septicemia (22.2% vs. 5.9% and 8.3%; Pc < 0.001) were more frequent in patients with severe NK cell lymphopenia than in patients from the other two groups. Streptococcus pneumoniae was documented in 28 patients from the severe NK cell lymphopenia group, 26 patients from the mild NK cell lymphopenia group, and 43 patients from the group with normal NK cell counts (22.4%, 19% and 16.4%, respectively, of the pneumonia episodes in each group). Haemophilus influenzae was documented in 12 patients from the severe NK cell lymphopenia group, 10 patients from the mild NK cell lymphopenia group, and nine patients from the group of patients with normal NK cell counts (9.6%, 14.3% and 3.4%, respectively, of the pneumonia episodes in each group). Other pathogens documented during pneumonia episodes were less frequent and are detailed in Supplemental Table S3. The pathogens identified in cases of septicemia were gram-negative bacilli in 7 (35% of septicemia episodes in the severe NK cell lymphopenia group), 3 (42.9% of septicemia episodes in the mild NK cell lymphopenia group) and 5 (26.3% of septicemia episodes in the group of patients with normal NK cell counts) patients, and gram-positive cocci were identified in 13 (65% of septicemia episodes), 4 (57.1%) and 14 (73.7%) patients from the severe NK cell lymphopenia, mild NK cell lymphopenia and normal NK cell count groups, respectively.
Fig. 3.
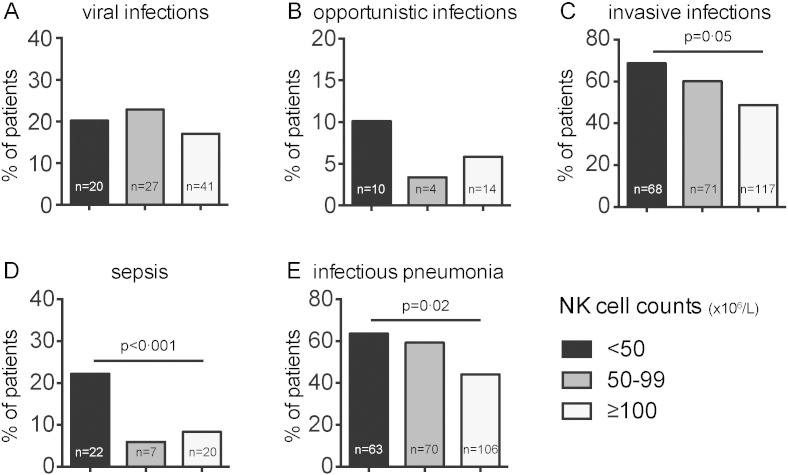
Proportion of CVID patients with different types of infection, as a function of NK cell count. Percentage of patients with A. viral infections, B. opportunistic infections, C. invasive infections, D. sepsis, E. infectious pneumonia. In black, patients with severe NK cell deficiency; in grey, with mild NK cell lymphopenia; and in white, patients with normal NK cell counts. The statistical tests were all two-tailed and the P values were adjusted for multiple testing, by Benjamini-Hochberg procedure.
Immunoglobulin (Ig) replacement therapy at inclusion in the DEFI study was administered to 86.9% of patients with severe NK cell lymphopenia, 75.2% of patients with mild NK cell lymphopenia and 65.4% of patients with normal NK cell counts (Pc < 0.001). Nine patients (9.1%) in the group with severe NK cell lymphopenia, 5 patients (4.3%) in the group with mild NK cell lymphopenia and 24 patients (10%) in the group with normal NK cell counts underwent splenectomy. Corticosteroids had been used in 15 patients (15.2%) with severe NK cell lymphopenia, 13 patients (11.4%) with mild NK cell lymphopenia and 21 patients (8.9%) with normal NK cell counts. Other immunosuppressive treatments were less frequently prescribed and were found in only two patients from the severe NK cell lymphopenia group (2%), three patients from the mild NK cell lymphopenia group (2.5%) and six 6 patients (2.5%) in the normal NK cell count group (Table 1).
3.2. Immunologic Evaluation
The results of the analyses of the principal lymphocyte subsets at inclusion, serum immunoglobulin levels at diagnosis, and comparisons between patients with severe NK cell deficiency and patients with mild or no NK cell lymphopenia are provided in Table 3.
Table 3. Lymphocyte subsets at inclusion in the DEFI study and immunoglobulin levels at diagnosis, for patients with CVID and severe, mild or no NK cell lymphopenia.
Biological characteristics | NK < 50 | NK 50–99 | NK ≥ 100 | Reference values | Pc |
|---|---|---|---|---|---|
Total lymphocytes (× 106/L) | 967 [693–1187] | 1331 [1030–1739] | 1629 [1293–2236] | 1922 [1524–2503] | 0.003 |
CD3 (× 106/L) | 801 [548–1029] | 1079 [818–1436] | 1204 [939–1657] | 1419 [1122–1774] | 0.003 |
CD4 (× 106/L) | 415 [277–571] | 568 [380–779] | 691 [495–927] | 912 [682–1153] | 0.003 |
CD4 < 200 × 106/L | 8/99 (8.1) | 8/118 (6.8) | 9/240 (3.8) | – | ns |
Naive CD4 (× 106/L) | 63 [20–132] | 135 [41–246] | 183 [64–349] | 363 [295–550] | 0.003 |
Naive CD4 < 20 × 106/L | 25/99 (25.3) | 14/117 (12.0) | 23/237 (9.7) | – | 0.025 |
CD8 (× 106/L) | 310 [198–474] | 431 [339–598] | 479 [332–672] | 474 [356–616] | 0.003 |
CD19 (× 106/L) | 59 [19–116] | 99 [46–172] | 122 [69–209] | 202 [126–301] | 0.003 |
“no-B” (CD19 < 1%) | 16/97 (16.5) | 14/114 (12.3) | 18/233 (7.7) | – | ns |
CD19+ IgD− CD27+ (%) | 1 [0.5–3.0] | 2 [0.7–7.0] | 3 [1·0–9.0] | 14.5 [10.0–19.5] | 0.003 |
“SmB-” n (% of patients) | 55/97 (56.7) | 52/114 (45.6) | 84/233 (36.1) | – | ns |
IgG (at diagnosis) (g/L) | 1.98 [0.80–2.90] | 2 [0.72–3.54] | 3.42 [1.70–4.45] | NR: 6.72–12.48 | 0.003 |
IgA (at diagnosis) (g/L) | 0.09 [0.06–0·26] | 0.18 [0.07–0.34] | 0·26 [0.09–0.67] | NR: 1.00–3.18 | 0.003 |
IgM (at diagnosis) (g/L) | 0.19 [0.10–0·30] | 0.22 [0.10–0.42] | 0.3 [0.17–0.56] | NR: 0.55–1.55 | 0.005 |
Note. For absolute values and percentages of lymphocyte subsets and immunoglobulins, the median is shown [with 25th and 75th percentiles]. For lymphocyte subsets analysis, reference values correspond to the control population of 52 healthy blood donors. For immunoglobulins, reference values correspond to normal range (NR) of the laboratory. All P values were adjusted by Benjamini–Hochberg procedure (Pc); ns = not statistically significant.
Total lymphocyte counts were lower in patients with severe NK cell lymphopenia (median: 967 x106/L) than in patients with mild NK cell lymphopenia (median: 1331 × 106/L) or normal NK cell counts (median: 1629 × 106/L) (Pc = 0.003). Within the T-cell population, absolute numbers of CD4+ T cells were lower in patients with severe NK cell deficiency (median: 415 × 106/L) than in the other two groups (median: 568 × 106/L and 691 × 106/L, respectively) (Pc = 0.003). The counts of naïve CD4+ T cells (defined as CD4+ CD45RA+ CCR7+ cells) were also lower in patients with severe NK cell deficiency (median: 63 × 106/L) than in the other groups (135 × 106/L and 183 × 106/L, respectively) (Pc = 0.003). Severe CD4+ T-cell deficiency (CD4+ T cells < 200 × 106/L) affected 8·1% of the patients with severe NK cell deficiency, and severe naive CD4+ T-cell deficiency (naive CD4+ T cells < 20 × 106/L) (late-onset combined immunodeficiency or “LOCID” patients according to Malphettes and colleagues (Malphettes et al., 2009)) was found in 25.3% of the patients with severe NK cell deficiency (12.0% and 9.7% respectively in the 2 other groups) (Pc = 0.025).
Total B cell counts were also lower in patients with severe NK cell lymphopenia than in patients from the other two groups (median: 59 × 106/L vs. 99 × 106/L and 122 × 106/L) (Pc = 0.003). Furthermore, 16.5% of patients with severe NK cell lymphopenia had a CD19+ B cell count corresponding to ≤ 1% of the total lymphocyte count (B — group in the EuroClass classification (Wehr et al., 2008)), versus only 12.3% of patients with mild NK cell lymphopenia and 7.7% of patients with normal NK cell counts. Within the B-cell compartment, the median percentage of IgD− CD27+ switched memory B cells was 1%, 2% and 3% of B cells in patients with severe, mild and no NK cell deficiency, respectively. Expansion of CD21low B cells has been associated with lymphoproliferative and autoimmune manifestations during CVID. Number of CD21low B cells was available in n = 209 patients of our study and a correlation between CD21low B cells and NK cells numbers was observed (r = 0.13, P = 0.05).
Little is known about the stability of peripheral circulating NK cell counts in humans. We therefore checked the stability of NK cell counts over time in 35 patients from the principal center participating in the DEFI study (Saint-Louis Hospital, Paris). These patients underwent several phenotypic investigations, all carried out at the same reference laboratory, during follow-up. Ten of these 35 patients presented severe NK cell lymphopenia (< 50 × 106/L) at inclusion and, for 80% of these patients, NK cell counts remained below 100 × 106/L over time. For the 15 patients with NK cell counts > 100 × 106/L at inclusion, NK cell counts remained above 100 × 106/L over time in 73% (data not shown). Thus, NK cell lymphopenia and normal NK cell counts appeared to remain stable over time in most of these patients. Furthermore no correlation was observed between age at evaluation or age at diagnosis and NK cell count in our patients (data not shown).
Median serum immunoglobulin (Ig) G levels at diagnosis were lower in patients with severe and mild NK cell lymphopenia than in patients with normal NK cell counts (Pc = 0.003). IgA and IgM levels at diagnosis were also lower in patients with severe NK cell lymphopenia than in the other groups (Pc = 0.003 and Pc = 0.005 respectively).
3.3. Survival and Analysis of Subgroups Defined on the Basis of NK and CD4+ T Cell Counts
Survival five years after inclusion in the DEFI study was 86.7% for the severe NK cell lymphopenia group, 92.6% for the mild NK cell lymphopenia group and 92.4% for the group of patients with normal NK cell counts at inclusion (P = 0.16). Kaplan–Meier curves were plotted (Fig. 4A).
Fig. 4.
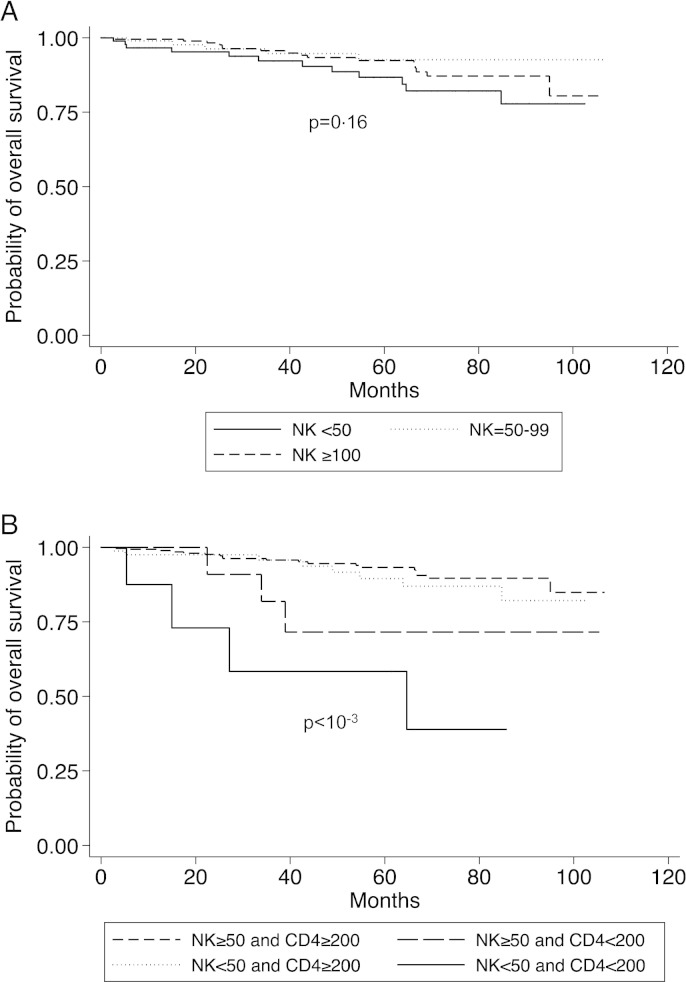
A. Overall survival in three groups of patients with CVID defined on the basis of NK cell counts (< 50, 50–99 and ≥ 100 × 106/L) (P = 0.16). B. Overall survival in four groups of patients with CVID defined on the basis of NK cell (< 50 and ≥ 50 × 106/L) and CD4+ T cell (< 200 and ≥ 200 × 106/L) counts (P < 10− 3). All statistical tests were two-tailed.
Low CD4+ T cell count is known to be associated with higher rates of mortality and clinical events in patients with CVID (Malphettes et al., 2009), and patients with severe NK-cell deficiency also have lower CD4+ T cell counts in our study. The cut-off of 200 × 106/L CD4+ T cells was based on the results of a previous work showing that a peculiar phenotype of “LOCID” (Late Onset Combined ImmunoDeficiency) was observed in CVID patients with CD4+ T cells below this value and on the basis of 2014 ESID definition of CVID patients with profound T-cell defect (Ameratunga et al., 2014). We studied survival and clinical events in four subgroups of patients defined on the basis of NK and CD4+ T cell counts (Supplemental Fig. S2): a “T − NK −” group (8 patients with both CD4+ T cell counts < 200 × 106/L and NK cell counts < 50 × 106/L), a “T − NK +” group (18 patients with CD4+ T cells < 200 × 106/L and NK cells ≥ 50 × 106/L), a “T + NK −” group (91 patients with CD4+ T cells ≥ 200 × 106/L and NK cells < 5 × 106/L) and a “T + NK +” group (351 patients with both CD4+ T cells ≥ 200 × 106/L and NK cells ≥ 50 × 106/L). Survival in these four subgroups of CVID patients is presented in Fig. 4B. Mortality was particularly high in the subgroup of patients with both NK cell and CD4+ T cell deficiencies (the “T − NK −” group), as survival at 5 years was 93.2%, 89.5%, 71.6% and 58.3% for the “T + NK +”, “T + NK −“, “T − NK +” and “T − NK −“subgroups, respectively (P < 0.0001). The prevalence of septicemia (Fig. 5) and pneumonia was higher in the “T − NK −” (50% of patients for septicemia and 87.5% of patients for pneumonia) and “T + NK −” (19.8% of patients for septicemia and 61.5% of patients for pneumonia) subgroups, and lower in the “T − NK +” (5.6% and 61.1%, respectively) and “T + NK +” (7.4% and 48.7% respectively) subgroups. Other disease-related complications (non-infectious clinical events) were observed in respectively 4/8 patients in the “T − NK −“ group (50.0%), 11/18 patients in the “T − NK +” group (61.1%), 52/91 patients in the “T + NK −“ group (57.1%) and 120/351 patients in the “T + NK + ” group (34.2%).
Fig 5.
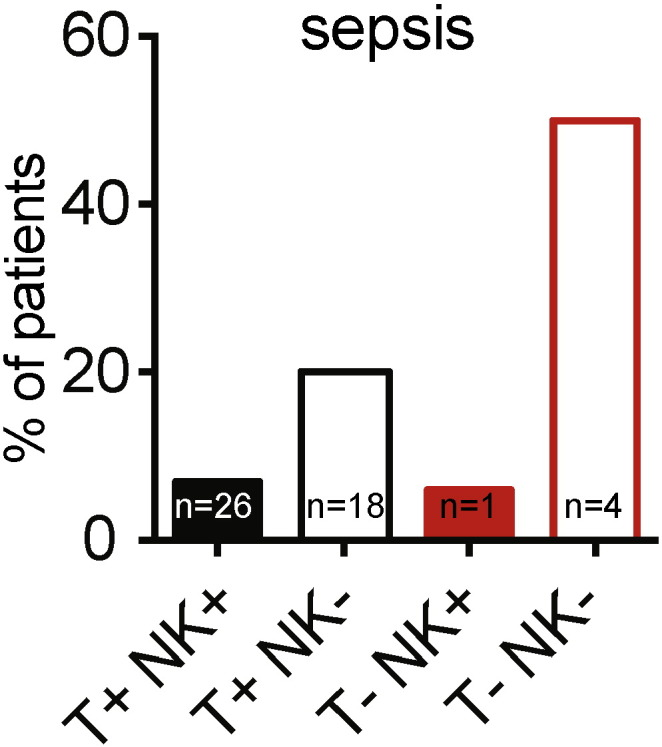
Proportion of patients with sepsis in four subgroups of CVID patients defined on the basis of NK cell (< 50 and ≥ 50 × 106/L) and CD4+ T cells (< 200 and ≥ 200 × 106/L) counts.
4. Discussion
Large cohorts for studying the correlation between NK cell deficiency and clinical events in humans are extremely rare. The study by Imai and colleagues (Imai et al., 2000) reporting correlation between low NK cell cytotoxicity and the incidence of cancer in a cohort of 3625 patients is the only such study published to date. Our study is original in addressing the role of NK cells in a large cohort of immunodeficient patients through exploration of the phenotype of patients with quantitative NK cell deficiency.
Our decision to study a cohort of patients with immunodeficiency is justified by the conclusion of a probable redundant role of these cells in humans reported by some studies in patients with “selective” NK cell deficiency. Studies of NK cell deficiency in patients with concomitant defect of one or several components of adaptive immunity, as in CVID patients, could thus reveal redundant in vivo functions of NK cells in humans.
Different classifications and subgroups of CVID patients have been proposed, defined on the basis of the quantitative abnormalities of B cells and T cells (Malphettes et al., 2009, Wehr et al., 2008), but data regarding the NK cell compartment of these patients and the phenotype of CVID patients with NK cell deficiency are rare. Few studies have already evaluated number of NK cells in CVID patients and observed a decreased number of NK cells in these patients (Aspalter et al., 2000, Berrón-Ruiz et al., 2014, Kutukculer et al., 2015). Nevertheless, our study is unique in reporting clinical manifestations and phenotype of patients with NK cell deficiency, especially in a cohort as large as more than 450 CVID patients. In 2000 Aspalter and colleagues reported a decrease in absolute and relative numbers of NK cells (defined as CD3− CD16+ cells) in 55 CVID patients compared to healthy controls, but without study of clinical manifestations associated with this NK cell deficiency (Aspalter et al., 2000). In 2015 Kutukculer and colleagues explored innate immunity in a small cohort of 20 pediatric CVID patients, with decreased percentage and increased cytotoxicity of NK cells in patients with severe disease (depending on the presence of splenomegaly, granuloma and/or bronchiectasis), but without detail about each of these clinical manifestations and other complications of the disease (Kutukculer et al., 2015). In the study of Aspalter et al., a decrease of invariant NK T cells has also been found in CVID patients, with again difficulties to conclude without available clinical data in this study (Aspalter et al., 2000). Finally, type 1 innate lymphoid cells (ILCs) can share phenotypical markers, such as CD56, with bona fide NK cells (Hazenberg and Spits, 2014) and a type 1 ILCs defect could potentially contribute to the observed phenotype of patients with NK cell deficiency.
Interestingly, although there is a large body of evidence concerning the role of NK cells in defense against viral pathogens (Lugli et al., 2014, Della Chiesa et al., 2014, Lee et al., 2007), the proportion of patients with viral infections did not differ between groups defined on the basis of their circulating NK cell counts in this large population of CVID patients. We also found no qualitative evidence for susceptibility to a particular type of virus in patients with severe NK cell lymphopenia. For example, Human papilloma virus (HPV) infections were equally frequent in all groups of patients, despite reports implicating NK cells in the control of infections with this pathogen (Kamili et al., 2014, Bere et al., 2014). Similarly, CMV infections did not appear to be more frequent in patients with severe NK cell deficiency than in patients with mild or no NK cell deficiency in our study. Difficult formal diagnosis of viral pathogens in clinical practice in these hypogammaglobulinemic patients, underestimation of benign viral episodes, or compensation by T cell compartment in this adult population could be as many explanations for this apparent discrepancy between earlier reports and our present study. In any event, these data highlight that the demonstration of a non-redundant role of NK cells in the control of viral infections has been obtained in the mouse, but not formally in humans.
The role of NK cells in anti-tumour responses has been extensively studied, for various types of tumour, in animal models and humans (Vivier et al., 2012, Shafer et al., 2013, Velardi et al., 2012). Imai and colleagues reported that low peripheral NK cell cytotoxicity was associated with a higher incidence of cancer (Imai et al., 2000). Surprisingly, in our study comparing CVID patients with different counts of peripheral NK cells, no differences between groups were observed for the incidence of neoplasms, including solid tumours and lymphomas. The median time from the onset of symptoms and evaluation in this study was 18 to 24.3 years in the groups studied, and the duration of follow-up would also be unlikely to account for this lack of association. The relatively young age at evaluation (median: from 40.5 to 44.8 years, depending on the group considered) in the DEFI study may account for the missing cancers, as many tumours occur after the age of 50 years (most of the events in the study by Imai and colleagues) (Imai et al., 2000).
The main finding in this study was the high frequency of invasive bacterial infections in CVID patients with severe NK cell lymphopenia (68.7%). This frequency was higher than that in patients with mild (60.2%) or no NK cell lymphopenia (48.8%). This higher incidence of severe bacterial infections was accounted for by a higher incidence of pneumonia and sepsis/bacteremia in particular, with bacteremia being found in 22.2% of patients with severe NK cell lymphopenia (versus only 5.9% and 8.3% of the patients in the other two groups). This higher incidence of severe bacterial infection did not seem to be related to the proportion of splenectomised patients, similar in the 3 studied groups, or to be related to an associated CD4+ T cell deficiency, because it persisted in the NK − T + group, particularly for sepsis/bacteremia. Several studies have reported protective or deleterious roles of NK cells in various models of bacterial infectious diseases. A protective role for NK cells was demonstrated for infections with Listeria monocytogenes, Mycobacterium avium, Mycobacterium tuberculosis and various strains of Salmonella (Katz et al., 1990, Harshan and Gangadharam, 1991, Bermudez et al., 1990, Wherry et al., 1991, Vankayalapati et al., 2005). Similarly, NK cells have been shown to play a critical protective role in septic arthritis and models of pulmonary infection with Staphylococcus aureus (Nilsson et al., 1999, Small et al., 2008). The mildly protective role of NK cells in these bacterial infections is thought to involve IFN-γ secretion, promoting macrophage phagocytic functions and/or the lysis of infected macrophages through the recognition of as yet unidentified activation ligands (Souza-Fonseca-Guimaraes et al., 2012). By contrast, deleterious effects of NK cell activation were reported after infection with gram-negative bacteria such as with Escherichia coli (Badgwell et al., 2002) or gram-positive bacteria such as with S. pneumoniae (Kerr et al., 2005). In these conditions, NK cells have been shown to contribute to septic shock (Barkhausen et al., 2008, Carson et al., 1999, Emoto et al., 2002, Heremans et al., 1994), but their depletion in S. pneumoniae-infected mice has also been reported to impair bacterial clearance (Elhaik-Goldman et al., 2011). The dual role of NK cells, which may be initially beneficial, helping to control bacterial load, but may then become deleterious in the acute phase of sepsis, mediating tissue damage, requires further investigation.
The prevalence of non-infectious disease-related complications, such as granuloma in particular, was also found to be higher in patients with severe NK cell lymphopenia than in patients with mild or no NK cell lymphopenia. In a description of 59 patients with granulomatous disease and CVID from the DEFI study, patients with granulomatous disease had lower NK cell counts (median: 60 × 106/L) than patients without granulomatous disease (median: 109 × 106/L) (Boursiquot et al., 2013). NK cell counts were not available in other series of patients with granulomatous disease and CVID (Ardeniz and Cunningham-Rundles, 2009, Bouvry et al., 2013, Mullighan et al., 1997). The etiology and mechanisms involved in granuloma in patients with CVID have yet to be elucidated. Granuloma may be a consequence of a persistent but undetected infection with a virus or another microorganism, leading to weaker cell-mediated, and possibly NK cell immunity. Consistent with this hypothesis, Bodemer and colleagues recently described the long-term persistence of live rubella virus vaccine as an antigenic trigger of cutaneous granulomas in patients with primary immunodeficiency (Bodemer et al., 2014). Advanced DNA sequencing technologies might be able to detect the presence in granuloma lesions of microbes not detected in conventional in vitro growth assays.
Although the first of its kind, our study has some limitations. Firstly, our results are based on clinical observations and measurements, with mostly retrospective data collection, and a statistical correlation cannot be interpreted as indicative of a causal relationship. Given the exploratory nature of this study, we carried out multiple hypothesis testing using the Benjamini–Hochberg method to control for false discovery rate. However these results should be interpreted with caution, and we cannot conclude that all significances are really cases, but most rejected hypotheses (e.g., around 95%) correspond to cases where the alternative hypothesis is true. The stability of NK cell counts in these patients over time is also a non-resolved issue.
Another key point relates to the lower counts of CD4+ T cells, naïve CD4+ T cells and B cells in the group of patients with severe NK cell lymphopenia. Indeed, severe CD4+ T cell deficiency and naïve CD4+ T cell deficiency have been associated with the “LOCID” phenotype, with a high frequency of non-infectious disease-related complications and opportunistic infections (Malphettes et al., 2009). Similarly, the “no-B” phenotype (B cells < 1%) and the presence of dysregulated B-cell subpopulations (low proportions of switched memory B cells, high transitional and CD21low B cell counts) are associated with clinical complications, such as splenomegaly, lymphadenopathy and granuloma (Wehr et al., 2008). In this line, higher rates of invasive infections and non-infectious complications observed in patients with NK cell deficiency could be explained by global, T and B cell lymphopenia observed in these patients. Nevertheless, patients with the “no-B″ phenotype, severe CD4+ T cell deficiencies (< 200 × 106/L) and very low naïve CD4+ T cell counts (< 20 × 106/L) accounted for “only” 16.5%, 8.1% and 25.3%, respectively, of the patients with severe NK cell deficiency, suggesting that these factors alone cannot account for the observed phenotype of patients with severe NK cell lymphopenia. The persistence of a higher frequency of pneumonia and bacteremia in the T + NK − subgroup also suggests that NK cells could contribute to this phenotype. Although the coexistence of T cell and B cell deficiencies associated in these patients introduce some difficulties in interpreting the clinical manifestations observed, we are convinced that it is in these “non-redundant” conditions of associated adaptive immune system deficiency that NK cell deficiency could be clinically relevant. Unfortunately, to show strictly and statistically this point, comparison should be performed between T −(B −)NK − and T −(B −)NK + groups, but these groups are the smallest groups (most uncommon patients) within our large cohort of CVID patients, with only respectively 8 and 18 patients. These numbers do not enable us to make stringent statistical analysis on the independent role of NK cells. Finally, the coexistence of T, B and NK cell deficiencies in some patients with CVID raises questions about the possibility of an as yet unidentified genetic defects leading to global lymphopoietic deficiencies in at least some of these patients (Ochs, 2014).
Mortality was not significantly higher in patients with severe NK cell deficiency than in patients with mild or no NK cell deficiency in our study. Nevertheless, mortality was particularly high (41.7% at 5 years) in the subgroup of patients with both severe NK cell lymphopenia and a severe CD4+ T cell deficiency. This suggests that NK cells could play a non-redundant role when the adaptive immune system, and the CD4 compartment in particular, is not optimal. Another recent study in 40 patients with idiopathic CD4 lymphopenia reported similar conclusions, with a correlation between low peripheral NK cell counts, and a high frequency of infections and low survival in these patients (Régent et al., 2014). In the same line, a recent study in five unrelated children with inherited DOCK2 deficiency, leading to T-cell, B-cell, and NK-cell defective responses, found early-onset invasive bacterial infections in these patients (Dobbs et al., 2015).
In conclusion, this large cohort study of CVID patients, despite some limitations, provides new insight into the functions of NK cells in vivo. Patients with CVID and NK cell deficiency seem to have a particular phenotype, with high frequencies of severe bacterial infections (pneumonia and bacteremia), and non-infectious disease-related complications, such as granuloma in particular. These data suggest that we should reconsider the role of human NK cells in controlling non-viral infections, such as extracellular bacterial infections in particular. The high frequency of these pathological events in this group of patients, and the high mortality of the T − NK − subgroup suggest that NK cells may have non-redundant immune functions in humans when the adaptive immune response is not optimal, and that reciprocally NK cells exert redundant functions in natura in normal individuals, at least in adults. This observation and the absence of a specific phenotype in SCID patients displaying a failure to reconstitute the NK compartment after HSCT suggest that there may be cooperation between T cells and NK cells, with NK cells helping to protect against pathogenic bacteria in the absence of T cells but becoming redundant if T cells are present. Several advantages are associated with redundant mechanisms. First, they can provide robust fail-safe mechanisms that ensure adequate protection (Nish and Medzhitov, 2011). Second, they can also limit the risk of immunopathologies, by ensuring the limited activation of each lymphocyte subset on challenge (Nish and Medzhitov, 2011). Our results thus pave the way for dissecting in more detail the interplay between NK cells, T cells and B cells in the control of bacterial infections in natura.
References
Ameratunga R., Brewerton M., Slade C., et al. Comparison of diagnostic criteria for common variable immunodeficiency disorder. Front. Immunol. 2014;5:415. doi: 10.3389/fimmu.2014.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ardeniz O., Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Clin. Immunol. 2009;133(2):198–207. doi: 10.1016/j.clim.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aspalter R.M., Sewell W.A., Dolman K., Farrant J., Webster A.D. Deficiency in circulating natural killer (NK) cell subsets in common variable immunodeficiency and X-linked agammaglobulinaemia. Clin. Exp. Immunol. 2000;121(3):506–514. doi: 10.1046/j.1365-2249.2000.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Badgwell B., Parihar R., Magro C., Dierksheide J., Russo T., Carson W.E., III Natural killer cells contribute to the lethality of a murine model of Escherichia coli infection. Surgery. 2002;132(2):205–212. doi: 10.1067/msy.2002.125311. [DOI] [PubMed] [Google Scholar]
Barkhausen T., Frerker C., Pütz C., et al. Depletion of NK cells in a murine polytrauma model is associated with improved outcome and a modulation of the inflammatory response. Shock. 2008;30(4):401–410. doi: 10.1097/SHK.0b013e31816e2cda. [DOI] [PubMed] [Google Scholar]
Bere A., Tayib S., Kriek J.M., et al. Altered phenotype and function of NK cells infiltrating human papillomavirus (HPV)-associated genital warts during HIV infection. Clin. Immunol. 2014;150(2):210–219. doi: 10.1016/j.clim.2013.12.005. [DOI] [PubMed] [Google Scholar]
Bermudez L.E., Kolonoski P., Young L.S. Natural killer cell activity and macrophage-dependent inhibition of growth or killing of Mycobacterium avium complex in a mouse model. J. Leukoc. Biol. 1990;47(2):135–141. doi: 10.1002/jlb.47.2.135. [DOI] [PubMed] [Google Scholar]
Berrón-Ruiz L., López-Herrera G., Vargas-Hernández A., et al. Lymphocytes and B-cell abnormalities in patients with common variable immunodeficiency (CVID) Allergol. Immunopathol. (Madr.) 2014;42(1):35–43. doi: 10.1016/j.aller.2012.07.016. [DOI] [PubMed] [Google Scholar]
Bodemer C., Sauvage V., Mahlaoui N., et al. Live rubella virus vaccine long-term persistence as an antigenic trigger of cutaneous granulomas in patients with primary immunodeficiency. Clin. Microbiol. Infect. 2014;20(10):O656–O663. doi: 10.1111/1469-0691.12573. [DOI] [PubMed] [Google Scholar]
Boursiquot J.N., Gérard L., Malphettes M., et al. Granulomatous disease in CVID: retrospective analysis of clinical characteristics and treatment efficacy in a cohort of 59 patients. J. Clin. Immunol. 2013;33(1):84–95. doi: 10.1007/s10875-012-9778-9. [DOI] [PubMed] [Google Scholar]
Bouvry D., Mouthon L., Brillet P.Y., et al. Granulomatosis-associated common variable immunodeficiency disorder: a case–control study versus sarcoidosis. Eur. Respir. J. 2013;41(1):115–122. doi: 10.1183/09031936.00189011. [DOI] [PubMed] [Google Scholar]
Carson W.E., Yu H., Dierksheide J., et al. A fatal cytokine-induced systemic inflammatory response reveals a critical role for NK cells. J. Immunol. 1999;162(8):4943–4951. [PubMed] [Google Scholar]
Casey J.P., Nobbs M., McGettigan P., Lynch S., Ennis S. Recessive mutations in MCM4/PRKDC cause a novel syndrome involving a primary immunodeficiency and a disorder of DNA repair. J. Med. Genet. 2012;49(4):242–245. doi: 10.1136/jmedgenet-2012-100803. [DOI] [PubMed] [Google Scholar]
Chapel H., Lucas M., Lee M., et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112(2):277–286. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
Chapel H., Lucas M., Patel S., et al. Confirmation and improvement of criteria for clinical phenotyping in common variable immunodeficiency disorders in replicate cohorts. J. Allergy Clin. Immunol. 2012;130(5):1197–1198.e9. doi: 10.1016/j.jaci.2012.05.046. [DOI] [PubMed] [Google Scholar]
Conley M.E., Notarangelo L.D., Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin. Immunol. 1999;93(3):190–197. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
Della Chiesa M., Marcenaro E., Sivori S., Carlomagno S., Pesce S., Moretta A. Human NK cell response to pathogens. Semin. Immunol. 2014;26(2):152–160. doi: 10.1016/j.smim.2014.02.001. [DOI] [PubMed] [Google Scholar]
Dobbs K., Domínguez Conde C., Zhang S.Y., et al. Inherited DOCK2 deficiency in patients with early-onset invasive infections. N. Engl. J. Med. 2015;372(25):2409–2422. doi: 10.1056/NEJMoa1413462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elhaik-Goldman S., Kafka D., Yossef R., et al. The natural cytotoxicity receptor 1 contribution to early clearance of Streptococcus pneumoniae and to natural killer-macrophage cross talk. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emoto M., Miyamoto M., Yoshizawa I., et al. Critical role of NK cells rather than V alpha 14(+)NKT cells in lipopolysaccharide-induced lethal shock in mice. J. Immunol. 2002;169(3):1426–1432. doi: 10.4049/jimmunol.169.3.1426. [DOI] [PubMed] [Google Scholar]
From the Centers for Disease Control and Prevention 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA. 1993;269(6):729–730. [PubMed] [Google Scholar]
Gineau L., Cognet C., Kara N., et al. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J. Clin. Invest. 2012;122(3):821–832. doi: 10.1172/JCI61014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harshan K.V., Gangadharam P.R. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infect. Immun. 1991;59(8):2818–2821. doi: 10.1128/iai.59.8.2818-2821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hazenberg M.D., Spits H. Human innate lymphoid cells. Blood. 2014;124(5):700–709. doi: 10.1182/blood-2013-11-427781. [DOI] [PubMed] [Google Scholar]
Heremans H., Dillen C., van Damme J., Billiau A. Essential role for natural killer cells in the lethal lipopolysaccharide-induced Shwartzman-like reaction in mice. Eur. J. Immunol. 1994;24(5):1155–1160. doi: 10.1002/eji.1830240522. [DOI] [PubMed] [Google Scholar]
Hughes C.R., Guasti L., Meimaridou E., et al. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J. Clin. Invest. 2012;122(3):814–820. doi: 10.1172/JCI60224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imai K., Matsuyama S., Miyake S., Suga K., Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356(9244):1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
Jouanguy E., Gineau L., Cottineau J., Béziat V., Vivier E., Casanova J.L. Inborn errors of the development of human natural killer cells. Curr. Opin. Allergy Clin. Immunol. 2013;13(6):589–595. doi: 10.1097/ACI.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamili Q.U., Seeborg F.O., Saxena K., et al. Severe cutaneous human papillomavirus infection associated with natural killer cell deficiency following stem cell transplantation for severe combined immunodeficiency. J. Allergy Clin. Immunol. 2014;134(6):1451–1453.e1. doi: 10.1016/j.jaci.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz P., Yeager H., Jr., Whalen G., Evans M., Swartz R.P., Roecklein J. Natural killer cell-mediated lysis of Mycobacterium-avium complex-infected monocytes. J. Clin. Immunol. 1990;10(1):71–77. doi: 10.1007/BF00917500. [DOI] [PubMed] [Google Scholar]
Kerr A.R., Kirkham L.A., Kadioglu A., et al. Identification of a detrimental role for NK cells in pneumococcal pneumonia and sepsis in immunocompromised hosts. Microbes Infect. 2005;7(5–6):845–852. doi: 10.1016/j.micinf.2005.02.011. [DOI] [PubMed] [Google Scholar]
Kutukculer N., Azarsiz E., Karaca N.E., et al. A clinical and laboratory approach to the evaluation of innate immunity in pediatric CVID patients. Front. Immunol. 2015;6:145. doi: 10.3389/fimmu.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee S.H., Miyagi T., Biron C.A. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28(6):252–259. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
Lugli E., Marcenaro E., Mavilio D. NK cell subset redistribution during the course of viral infections. Front. Immunol. 2014;5:390. doi: 10.3389/fimmu.2014.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malphettes M., Gérard L., Carmagnat M., et al. Late-onset combined immune deficiency: a subset of common variable immunodeficiency with severe T cell defect. Clin. Infect. Dis. 2009;49(9):1329–1338. doi: 10.1086/606059. [DOI] [PubMed] [Google Scholar]
Mullighan C.G., Fanning G.C., Chapel H.M., Welsh K.I. TNF and lymphotoxin-alpha polymorphisms associated with common variable immunodeficiency: role in the pathogenesis of granulomatous disease. J. Immunol. 1997;159(12):6236–6241. [PubMed] [Google Scholar]
Neven B., Leroy S., Decaluwe H., et al. Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood. 2009;113(17):4114–4124. doi: 10.1182/blood-2008-09-177923. [DOI] [PubMed] [Google Scholar]
Nilsson N., Bremell T., Tarkowski A., Carlsten H. Protective role of NK1.1 + cells in experimental Staphylococcus aureus arthritis. Clin. Exp. Immunol. 1999;117(1):63–69. doi: 10.1046/j.1365-2249.1999.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nish S., Medzhitov R. Host defense pathways: role of redundancy and compensation in infectious disease phenotypes. Immunity. 2011;34(5):629–636. doi: 10.1016/j.immuni.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ochs H.D. Common variable immunodeficiency (CVID): new genetic insight and unanswered questions. Clin. Exp. Immunol. 2014;178(Suppl. 1):5–6. doi: 10.1111/cei.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oksenhendler E., Gérard L., Fieschi C., et al. Infections in 252 patients with common variable immunodeficiency. Clin. Infect. Dis. 2008;46(10):1547–1554. doi: 10.1086/587669. [DOI] [PubMed] [Google Scholar]
Régent A., Autran B., Carcelain G., et al. Idiopathic CD4 lymphocytopenia: clinical and immunologic characteristics and follow-up of 40 patients. Medicine (Baltimore) 2014;93(2):61–72. doi: 10.1097/MD.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shafer D., Smith M.R., Borghaei H., et al. Low NK cell counts in peripheral blood are associated with inferior overall survival in patients with follicular lymphoma. Leuk. Res. 2013;37(10):1213–1215. doi: 10.1016/j.leukres.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Small C.L., McCormick S., Gill N., et al. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J. Immunol. 2008;180(8):5558–5568. doi: 10.4049/jimmunol.180.8.5558. [DOI] [PubMed] [Google Scholar]
Souza-Fonseca-Guimaraes F., Parlato M., Philippart F., Misset B., Cavaillon J.M., Adib-Conquy M. Captain study group. Toll-like receptors expression and interferon-γ production by NK cells in human sepsis. Crit. Care. 2012;16(5):R206. doi: 10.1186/cc11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spits H., Artis D., Colonna M., et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
Vankayalapati R., Garg A., Porgador A., et al. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J. Immunol. 2005;175(7):4611–4617. doi: 10.4049/jimmunol.175.7.4611. [DOI] [PubMed] [Google Scholar]
Velardi A., Ruggeri L., Mancusi A. Killer-cell immunoglobulin-like receptors reactivity and outcome of stem cell transplant. Curr. Opin. Hematol. 2012;19(4):319–323. doi: 10.1097/MOH.0b013e32835423c3. [DOI] [PubMed] [Google Scholar]
Vivier E., Raulet D.H., Moretta A., et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vivier E., Ugolini S., Blaise D., Chabannon C., Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012;12(4):239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wehr C., Kivioja T., Schmitt C., et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111(1):77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
Wherry J.C., Schreiber R.D., Unanue E.R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect. Immun. 1991;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]